CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
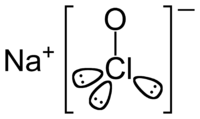
Sodium hypochlorite [1] |
|
Synonyms |
Hypochlorous acid, sodium salt, bleach, sodium hypochlorite aqueous solution, sodium hypochlorite solution, etc. [1] |
|
IUPAC name |
sodium hypochlorite[1] |
|
CAS No |
7681-52-9 |
|
REACH registration number |
|
|
EC No |
231-668-3 |
|
Molecular formula |
|
|
Substance group/chemical family |
Mono constituent substance/ Inorganic |
|
Appearance Physical state Odour Form Colour |
Liquid (100%) at 20°C and 1013 hPa watery liquid household bleach odour, disagreeable, sweetish odour [2] Greenish yellow [2] |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
Consumer uses: This substance is used in the following products: washing & cleaning products, textile treatment products and dyes, water treatment chemicals, perfumes and fragrances and cosmetics and personal care products. Biocidal uses: This substance is approved for use as a biocide in the EEA and/or Switzerland, for: human hygiene, disinfection, veterinary hygiene, food and animals feeds, drinking water. This substance is being reviewed for use as a biocide in the EEA and/or Switzerland, for: preservation for liquid systems, controlling slimes. Industrial uses: This substance is used in the following products: textile treatment products and dyes, washing & cleaning products, paper chemicals and dyes, water treatment chemicals and pH regulators and water treatment products. This substance has an industrial use resulting in manufacture of another substance (use of intermediates). This substance is used in the following areas: municipal supply (e.g. electricity, steam, gas, water) and sewage treatment. This substance is used for the manufacture of: chemicals, food products, textile, leather or fur and pulp, paper and paper products. This substance is used in the following activities or processes at workplace: transfer of chemicals, transfer of substance into small containers, mixing in open batch processes, closed processes with no likelihood of exposure, closed batch processing in synthesis or formulation, closed, continuous processes with occasional controlled exposure, batch processing in synthesis or formulation with opportunity for exposure and treatment of articles by dipping and pouring. [1] |
|
Handling considerations |
Prevention statementsWhen handling this substance: do not breathe the dust, fume, gas, mist, vapours or spray; avoid release to the environment; wear protective gloves and/or clothing, and eye and/or face protection as specified by manufacturer/supplier. Response statementsIn case of incident: If skin irritation occurs: Get medical advice/attention. If on skin (or hair): take off immediately all contaminated clothing. Rinse skin with water or shower. Immediately call a poison center or doctor/physician. Absorb spillage to prevent material damage. If in eyes: rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing. Storage statementsStore this substance in a well-ventilated place and keeping container tightly closed [1] |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
74.44 g/mol |
|
Bulk density/Specific gravity |
1.3 @ 21.2 °C [1], 1.06 at 20 °C (liquid) [2] |
|
pH |
|
|
Particle size |
|
|
EC |
|
|
Melting point |
-28.9 °C @ 101.3 kPa [1] |
|
Boiling point |
111 °C in solution [2] |
|
Flash point |
111 °C @ 101.3 kPa [1] |
|
Flammability |
Sodium hypochlorite solution is a weak base that is inflammable. [1] |
|
Vapour density |
|
|
Vapour pressure |
2 - 2.5 kPa at 20 °Cn [2] |
|
Solubility in water |
1 000 g/L @ 25 °C and pH 12 [1] 29.3 g/100 g (0 °C) in water [2] |
|
Solubility in organic solvents |
|
|
Solubility in inorganic solvents |
|
|
Hydrolysis |
|
|
Ionicity in water |
|
|
Surface tension |
82.4 mN/m @ 316 mg/L @ 20 °C [1] |
|
Dispersion properties |
|
|
Explosiveness |
Non-explosive (100%) [1] |
|
Other properties |
non oxidising [1] Dynamic viscosity at 20 °C: 6.4 mPa.s [1] |
|
Stability and reactivity |
|
|
Chemical stability |
Highly unstable [2] Chlorine evaporates at a rate of 0,75 gram active chlorine per day from the solution. Heated sodium hypochlorite disintegrates. This also happens when sodium hypochlorite comes in contact with acids, sunlight, certain metals and poisonous and corrosive gasses, including chlorine gas. [5] Strong oxidizing agent[2] Sodium hypochlorite is a strong oxidator and reacts with flammable compounds and reductors. [5] |
|
Reactivity hazards |
May decompose, generating irritating chlorine gas. [2] Decomposed by carbon dioxide from air. [2] |
|
Corrosivity |
|
|
Polimerization |
|
|
Incompatibility with various substances |
|
|
Special remarks on reactivity |
When heated to decomposition it emits toxic fumes of Na2O and hydrogen chloride. [2] |
|
Physical, chemical and biological coefficient |
|
|
Koc |
@ 20°C: 0.001 [1]
|
|
Kow |
Log Kow (Log Pow): -3.42 @ 20 °C [1] |
|
pKa |
|
|
log Kp |
|
|
Henry-constant |
0.076 Pa.m³.mol-1 @ 20 °C |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
|
|
General terrestrial fate |
|
|
General aquatic fate |
The substance is extremely reactive, any sodium hypochlorite that is poured into the drain from household use will react with organic matter and will be removed before reaching the environment. [4] |
|
General atmospheric fate |
Chlorine does not persist in the atmosphere either. [4] |
|
General persistence and degradability |
The substance is extremely reactive, any sodium hypochlorite that is poured into the drain from household use will react with organic matter and will be removed before reaching the environment. Chlorine does not persist in the atmosphere either. [4] |
|
Abiotic degradation and metabolites |
Regarding chlorinated by-products, the EU risk assessment considered tests on whole effluent from chlorinated raw sewage and observed that the halogenated by-products present did not increase the toxicity or reduce the biodegradability of the effluent. As this represents a “realistic worst case”, there should be no cause for concern for halogenated by-products generated by aqueous use of chlorine. The same is concluded for the atmospheric compartment. [4] |
|
Biodegradation and metabolites |
Sodium hypochlorite is rapidly degraded in presence of organic matter and therefore does not not bioaccumulate and does not persist in the environment. [4] |
|
Bioconcentration |
It does not bioaccumulate |
|
Volatilization |
|
|
Photolysis |
Phototransformation in air: Half life in air: 3.833 months Phototransformation in water: Dissipation Half-life (DT50): 12 - 60 min |
|
Hydrolysis |
|
|
Soil adsorption and mobility |
|
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
|
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems |
LC50: 60 µg/L (freshwater fish) [1] LC50: 32 µg/L (marine water fish) [1] EC50 / LC50: 35 µg/L (freshwater invertebrates) [1] EC50 / LC50: 26 µg/L (marine water invertebrates) [1] EC50: 49.9 µg/L (freshwater algae) [1] EC50: 100 µg/L (freshwater plants) [1] EC50: 77.1 mg/L (microorganisms) [1] Both sodium hypochlorite and chlorine do not deactivate Giardia Lambia and Cryptosporidium. [5] |
|
Terrestrial systems |
|
|
Chronic toxicity (NOEC, LOEC) |
|
|
Aquatic systems |
EC10 / LC10 or NOEC: 40 µg/L (marine water fish) [1] EC10 / LC10 or NOEC: 7 µg/L (marine water invertebrates) [1] EC10 or NOEC: 2.1 µg/L (freshwater algae) [1] EC10 or NOEC: 20 µg/L (freshwater plants) [1] EC10 or NOEC: 46.9 mg/L (microorganisms) [1] |
|
Terrestrial systems |
EC10 / LC10 / NOEC (70 days): 200 mg/kg food (birds) [1] |
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
inhalation of its aerosol, dermal, ingestion, eyes [2] |
|
General effects |
Serious local effects by all routes of exposure [2] |
|
Endocrine disruption |
|
|
Mutagenicity |
Chlorine is of no concern with regard to mutagenicity and carcinogenicity and none of the chlorination by-products studied to date is a carcinogen at concentrations normally found in drinking-water. [3] |
|
Carcinogenicity |
Chlorine is of no concern with regard to mutagenicity and carcinogenicity and none of the chlorination by-products studied to date is a carcinogen at concentrations normally found in drinking-water. In an exceptionally long (7 generations) rat toxicity study, the incidence of malignant tumours in the animals consuming drinking-water with a chlorine level of 100 mg/L did not differ from that in controls animals. However, discussions still remain for bladder cancer even if the evidence of an association, although the causal nature of the association remains inconclusive. [3] |
|
Reprotoxicity |
The Scientific Committee on Health and Environmental Risks (SCHER) of DG SANTE (EU Commission) supported the conclusion that, based on the available database on hypochlorite and chlorine, there presently is no evidence for developmental or reproductive toxicity of sodium hypochlorite. [4] |
|
Teratogenicity |
The Scientific Committee on Health and Environmental Risks (SCHER) of DG SANTE (EU Commission) supported the conclusion that, based on the available database on hypochlorite and chlorine, there presently is no evidence for developmental or reproductive toxicity of sodium hypochlorite. [4] |
|
Skin, eye and respiratory irritations |
Skin: Adverse effect observed (corrosive) Eye: Adverse effect observed (irritating) Respiratory: Adverse effect observed (irritating) [1] It has been reported that, in some conditions, particularly in young children, asthma can be triggered by exposure to chlorinated water, via the chloramines by-products, even if the scientific evidence does not clearly support that recreational swimming increases the risk of childhood asthma. Episodes of dermatitis have also been associated with exposure to chlorine and hypochlorite but all these elements were not yet discussed in the latest reference reports available.There has been some association with dermatitis, although the evidence has not been discussed in consensus reference documents. [4] |
|
Metabolism: absorption, distribution & excretion |
|
|
Exposure limits |
DNEL: 1.55 mg/m³ (workers, general population, inhalation, systemic effects, repeated dose toxicity, long term) DNEL: 3.1 mg/m³ (workers, general population, inhalation, systemic effects, repeated dose toxicity, acute short term) DNEL: 1.55 mg/m³ (workers, general population, inhalation, local effects, repeated dose toxicity, long term) DNEL: 3.1 mg/m³ (workers, general population, inhalation, local effects, repeated dose toxicity, acute short term) DNEL: 260 µg/kg bw/day (general population, oral, systemic effects, repeated dose toxicity, long term) [1] |
|
Drinking water MAC |
The guideline value for chlorine defined by the WHO is 5 mg/L. It should be noted that this value is conservative. Interestingly, most individuals are able to taste chlorine or its by-products (e.g. chloramines) at concentrations below 5 mg/L and some at levels as low as 0.3 mg/L. [4] |
|
Other information |
Skin sensitisation: No adverse effect observed (not sensitising) [1] |
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
LD50: 1 100 mg/kg bw (rat, oral) [1] (Study cannot be used for classification) LD50: 20 000 mg/kg bw (rabbit, dermal) |
|
Chronic toxicity (NOEL, LOEL) |
NOAEL: 50 mg/kg bw/day (subchronic, rat) oral route, systemic effect – repeated dose toxicity) [1] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
|
Danger! According to the harmonised classification and labelling (ATP13) approved by the European Union, this substance causes severe skin burns and eye damage, is very toxic to aquatic life, is very toxic to aquatic life with long lasting effects and causes serious eye damage. Additionally, the classification provided by companies to ECHA in REACH registrations identifies that this substance may be corrosive to metals, causes skin irritation and may cause respiratory irritation. [1]
According to REACH registrations: H400: Very toxic to aquatic life. H314: Causes severe skin burns and eye damage. H318: Causes serious eye damage. H290: May be corrosive to metals. H335: May cause respiratory irritation. H410: Very toxic to aquatic life with long-lasting effects. H411: Toxic to aquatic life with long-lasting effects. H315: Causes skin irritation. H319: Causes serious eye irritation. H412: Harmful to aquatic life with long-lasting effects.
According to CLP notifications: H400: Very toxic to aquatic life. H314: Causes severe skin burns and eye damage. H318: Causes serious eye damage. H290: May be corrosive to metals. H335: May cause respiratory irritation. H410: Very toxic to aquatic life with long-lasting effects. H411: Toxic to aquatic life with long-lasting effects. H315: Causes skin irritation. H319: Causes serious eye irritation. H412: Harmful to aquatic life with long-lasting effects. H336: May cause drowsiness or dizziness. |
|
|
EINECS regulation |
̵listed on EINECS (European INventory of Existing Commercial chemical Substances) List |
|
OSHA regulations etc. |
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
|
|
|
CREATED, LAST UPDATE |
|
|
Created |
2019. 11. 29 |
|
Last update |
2020. 09. 23 |
|
REFERENCES |
|
|
[1] ECHA, Sodium hypochlorite, https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.028.790, Accessed 2019.11.29 [2] PubChem, Sodium hypochlorite , https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-hypochlorite, Accessed 2019.12.02 [3] COC - UK Committee on Carcinogenicity of chemicals in food consumer products and the environment. Annual Report 2008 - Second Statement on Chlorinated Drinking Water and Cancer, https://www.greenfacts.org/en/chlorine-sodium-hypochlorite/index.htm, Accessed 2020.09.23 [4] Greenfacts. Facts on health and Environment, Report Highlights, Chlorine Sodium Hypochlorite, (last updated 2017) https://www.greenfacts.org/en/chlorine-sodium-hypochlorite/index.htm, Accessed 2020.09.23 [5] Lenntech, Water treatment solutions, Disinfection. https://www.lenntech.com/processes/disinfection/chemical/disinfectants-sodium-hypochlorite.htm Accessed 2020.09.23 |
|