CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
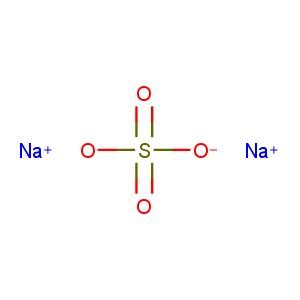
Sodium sulphate[1] |
|
Synonyms |
Sulfuric acid, sodium salt, Glauber's salt, Disodium sulfate decahydrate, Sodium Sulphate Anhydrous [1] |
|
IUPAC name |
disodium sulfate [1] |
|
CAS No |
7757-82-6 |
|
REACH registration number |
|
|
EC No |
231-820-9 |
|
Molecular formula |
Na2O4S [1] |
|
Substance group/chemical family |
Mono constituent substance/ Inorganic [1] |
|
Appearance Physical state Odour Form Colour |
Solid (100%) at 20°C and 1013 hPa [1]
Odourless (100%) [1] Crystalline (47%), Powder (46%), Solid: particulate/powder (7%) [1] |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
This substance is used in the following products: paper chemicals and dyes, textile treatment products and dyes and leather treatment products.This substance is used in the following areas: mining. This substance is used for the manufacture of: chemicals, textile, leather or fur, pulp, paper and paper products and plastic products.This substance is used in the following activities or processes at workplace: transfer of chemicals, transfer of substance into small containers, closed batch processing in synthesis or formulation, closed, continuous processes with occasional controlled exposure, mixing in open batch processes and laboratory work. [1] |
|
Handling considerations |
Prevention statementsWhen handling this substance: do not get in eyes, on skin, or on clothing. [1] |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
|
|
Bulk density/Specific gravity |
relative density: 2.7 g/cm³ @ 20 °C [1] |
|
pH |
|
|
Particle size |
|
|
EC |
|
|
Melting/Freezing point |
800 - 888 °C [1] |
|
Boiling point |
|
|
Flash point |
|
|
Flammability |
Non flammable (100%) |
|
Vapour density |
|
|
Vapour pressure |
|
|
Solubility in water |
445.5 g/L @ 20 °C [1] |
|
Solubility in organic solvents |
|
|
Solubility in inorganic solvents |
|
|
Hydrolysis |
|
|
Ionicity in water |
|
|
Surface tension |
71 mN/m @ 1 000 mg/L @ 20 °C [1] |
|
Dispersion properties |
|
|
Explosiveness |
Non-explosive (100%) [1] |
|
Other properties |
dynamic viscosity (in mPa s): 2.481 [1]
|
|
Stability and reactivity |
|
|
Chemical stability |
non-oxidising (100%) [1] |
|
Reactivity hazards |
|
|
Corrosivity |
|
|
Polimerization |
|
|
Incompatibility with various substances |
|
|
Special remarks on reactivity |
|
|
Physical, chemical and biological coefficient |
|
|
Koc |
|
|
Kow |
|
|
pKa |
|
|
log Kp |
|
|
Henry-constant |
|
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
Release to the environment of this substance can occur from industrial use: in the production of articles, in processing aids at industrial sites, as processing aid, as an intermediate step in further manufacturing of another substance (use of intermediates) and formulation of mixtures. Other release to the environment of this substance is likely to occur from: indoor use (e.g. machine wash liquids/detergents, automotive care products, paints and coating or adhesives, fragrances and air fresheners) and outdoor use. [1] |
|
General terrestrial fate |
|
|
General aquatic fate |
|
|
General atmospheric fate |
|
|
General persistence and degradability |
|
|
Abiotic degradation and metabolites |
|
|
Biodegradation and metabolites |
|
|
Bioconcentration |
|
|
Volatilization |
|
|
Photolysis |
|
|
Hydrolysis |
|
|
Soil adsorption and mobility |
|
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
|
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems |
LC50: 7960 mg/L (freshwater fish, 4 days) [1] EC50/LC50: 1766 mg/L (freshwater invertebrates, 48 hours) [1] EC50: 1.9 g/L (freshwater algae, 5 days) [1] |
|
Terrestrial systems |
|
|
Chronic toxicity (NOEC, LOEC) |
|
|
Aquatic systems |
EC10 / LC10 or NOEC: 1109 mg/L (marine invertebrates) [1] EC10 or NOEC: 8000 mg/L (microorganisms, 37 days) [1] |
|
Terrestrial systems |
|
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
|
|
General effects |
|
|
Endocrine disruption |
|
|
Mutagenicity |
|
|
Carcinogenicity |
|
|
Reprotoxicity |
|
|
Teratogenicity |
|
|
Skin, eye and respiratory irritations |
Skin: No adverse effect observed (not irritating), Skin sensitisation: No adverse effect observed (not sensitising) Eye: No adverse effect observed (not irritating) [1] |
|
Metabolism: absorption, distribution & excretion |
No bioaccumulation potential [1] |
|
Exposure limits |
DNEL: 20 mg/m³ (workers, inhalation, long term, systemic effects, repeated dose toxicity) [1] DNEL: 20 mg/m³ (workers, inhalation, long term, local effects, repeated dose toxicity) [1] DNEL: 12 mg/m³ (general population, inhalation, long term, systemic effects, repeated dose toxicity) [1] DNEL: 12 mg/m³ (general population, inhalation, long term, local effects, repeated dose toxicity) [1] |
|
Drinking water MAC |
|
|
Other information |
|
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
LD50 2 000 mg/kg bw (oral route , rat), [1] LD50 5 989 mg/kg bw (oral route, mouse) LD50 6 346 mL/kg bw (oral route, mouse)[1] LC50 (4 h): 2.4 mg/L air (inhalation, rat), Study cannot be used for classification [1] LC100 (60 min): 1.8 - 1.95 mg/m³ air (inhalation, rabbit), Study cannot be used for classification [1] |
|
Chronic toxicity (NOEL, LOEL) |
NOAEL (rat): 320 - 2 000 mg/kg diet (oral, repeated dose toxicity) [1] NOEL (rat): 1 000 mg/kg bw/day (oral, repeated dose toxicity) [1] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
| REACH/CLP |
According to the notifications provided by companies to ECHA in REACH registrations no hazards have been classified. According to the majority of notifications provided by companies to ECHA in CLP notifications no hazards have been classified [1] |
|
EINECS regulation |
̵listed on EINECS (European INventory of Existing Commercial chemical Substances) List |
|
OSHA regulations etc. |
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
CREATED, LAST UPDATE |
|
|
Created |
2019. 12. 06 |
|
Last update |
2019. 12. 06 |
|
REFERENCES |
|
|
[1] ECHA, Sodium sulphate, https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.028.928, Accessed 2019. 12. 06 |
|