CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
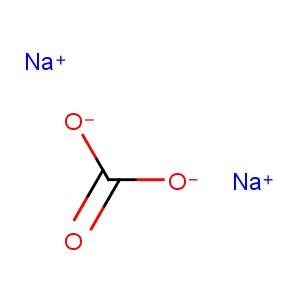
Sodium carbonate [1] |
|
Synonyms |
Soda ash, Calcined soda, Carbonic acid disodium salt, Sodium carbonate, anhydrous, Washing soda, etc. [2] |
|
IUPAC name |
disodium carbonate [1] |
|
CAS No |
497-19-8 |
|
REACH registration number |
|
|
EC No |
207-838-8 |
|
Molecular formula |
CNa2O3 [1] |
|
Substance group/chemical family |
Mono constituent substance/ Inorganic [1] |
|
Appearance Physical state Odour Form Colour |
Solid (100%) at 20°C and 1013 hPa [1]
Odourless (50%), Other (50% [1] Powder (100%) [1] or lumps [2] Greyish-white [2] |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
This substance is used in the following areas: mining. This substance is used for the manufacture of: chemicals and mineral products (e.g. plasters, cement). This substance is used in the following activities or processes at workplace: transfer of chemicals, closed, continuous processes with occasional controlled exposure, closed processes with no likelihood of exposure, closed batch processing in synthesis or formulation, batch processing in synthesis or formulation with opportunity for exposure, potentially closed industrial processing with minerals/metals at elevated temperature (e.g. smelters, furnaces, refineries, coke ovens) and transfer of substance into small containers. [1] |
|
Handling considerations |
Prevention statementsWhen handling this substance: wash parts of the body (as specified by manufacturer/supplier)in contact with substance thoroughly after handling; wear protective gloves and/or clothing, and eye and/or face protection as specified by manufacturer/supplier. Response statementsIn case of incident: If in eyes: rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing. If eye irritation persists get medical advice/attention. [1] |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
105.988 g/mol |
|
Bulk density/Specific gravity |
2.52 - 2.532 g/cm³ [1] |
|
pH |
Aqueous solutions are strongly alkaline [2] |
|
Particle size |
|
|
EC |
|
|
Melting/Freezing point |
851 °C at 101 325 Pa |
|
Boiling point |
|
|
Flash point |
|
|
Flammability |
|
|
Vapour density |
|
|
Vapour pressure |
|
|
Solubility in water |
|
|
Solubility in organic solvents |
Insoluble in ethanol, insoluble in acetone [2] |
|
Solubility in inorganic solvents |
|
|
Hydrolysis |
|
|
Ionicity in water |
|
|
Surface tension |
|
|
Dispersion properties |
|
|
Explosiveness |
|
|
Other properties |
|
|
Stability and reactivity |
|
|
Chemical stability |
Hygroscopic. Stable under recommended storage conditions. [2] |
|
Reactivity hazards |
|
|
Corrosivity |
|
|
Polimerization |
|
|
Incompatibility with various substances |
acids, acid salts cause its decomposition [2] |
|
Special remarks on reactivity |
|
|
Physical, chemical and biological coefficient |
|
|
Koc |
|
|
Kow |
|
|
pKa |
|
|
log Kp |
|
|
Henry-constant |
|
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
Sodium carbonate's production and use in manufacture of sodium salts, glass, soap; for washing wool; textiles; in bleaching linen, cotton; general cleanser; in water-softening; in photography; as reagent in analytical chemistry for pH adjustment; buffer; standard for acid-base titrimetry. Pharmaceutic aid (alkalinizer) will result in its release to the environment through various waste streams. Its use as a fungicide for use as hard surface disinfectants and sanitizers in institutional and residential settings and use in hydraulic fracking will result in its direct release to the environment [2] |
|
General terrestrial fate |
|
|
General aquatic fate |
|
|
General atmospheric fate |
|
|
General persistence and degradability |
|
|
Abiotic degradation and metabolites |
|
|
Biodegradation and metabolites |
|
|
Bioconcentration |
|
|
Volatilization |
|
|
Photolysis |
|
|
Hydrolysis |
|
|
Soil adsorption and mobility |
|
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
|
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems |
LC50: 300 mg/L (freshwater fish, 4 days) [1] EC50/LC50 (48 h): 200 mg/L (freshwater invertebrates) [1] |
|
Terrestrial systems |
|
|
Chronic toxicity (NOEC, LOEC) |
|
|
Aquatic systems |
|
|
Terrestrial systems |
|
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
oral, skin, respiratory, eye exposure |
|
General effects |
irritating |
|
Endocrine disruption |
|
|
Mutagenicity |
|
|
Carcinogenicity |
|
|
Reprotoxicity |
|
|
Teratogenicity |
|
|
Skin, eye and respiratory irritations |
Skin: No adverse effect observed (not irritating); Skin sensitisation: No adverse effect observed (not sensitising); Eye: Adverse effect observed (irritating); Respiratory: No study available [1] |
|
Metabolism: absorption, distribution & excretion |
No bioaccumulation potential [1] |
|
Exposure limits |
DNEL: 10 mg/m³ (workers, inhalation, long term, local effects, irritation (respiratory tract) [1] DNEL: 10 mg/m³ (general population, inhalation, acute/short term, local effects, irritation (respiratory tract) [1] Workers & general population, dermal, acute/short term, local effects, repeated dose toxicity: Low hazard (no threshold derived) [1] Workers & general population, eye exposure: Medium hazard (no threshold derived) [1] |
|
Drinking water MAC |
|
|
Other information |
|
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
LD50 2 800 mg/kg bw (oral route , rat), No adverse effect observed [1] LC50 2 300 mg/m³(inhalation, rat), No adverse effect observed [1] L50: 2 000 mg/kg bw (dermal, rabbit), No adverse effect observed [1] |
|
Chronic toxicity (NOEL, LOEL) |
|
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
| REACH/CLP |
Warning! According to the harmonised classification and labelling (CLP00) approved by the European Union, this substance causes serious eye irritation. [1] According to REACH registrations and CLP notifications: H319: Causes serious eye irritation. |
|
EINECS regulation |
̵listed on EINECS (European INventory of Existing Commercial chemical Substances) List |
|
OSHA regulations etc. |
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
CREATED, LAST UPDATE |
|
|
Created |
2019. 12. 05 |
|
Last update |
2019. 12. 05 |
|
REFERENCES |
|
|
[1] ECHA, Sodium carbonate, https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.007.127, Accessed 2019. 12. 05 [2] Pubchem, Sodium carbonate (compound) https://pubchem.ncbi.nlm.nih.gov/compound/10340#section=Ecotoxicity-Values, Accessed 2019. 12. 05 |
|