CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
Sulphuric acid |
|
Synonyms |
sulfur acid; oil of vitriol; mattling acid; battery acid; Dipping acid; acidum sulfuricum |
|
IUPAC name |
acetic acid; s. acid |
|
CAS No |
7664-93-9 |
|
REACH registration number |
− |
|
EC No |
231-639-5 |
|
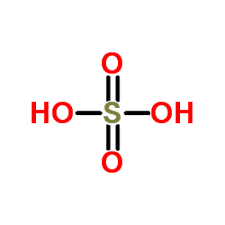
Molecular formula |
H2O4S [6] |
|
Substance group/chemical family |
Inorganic (100% )acid [6] |
|
Appearance Physical state Odour Form Colour |
Liquid (100%) at 20°C and 1013 hPa [6] Odourless
Colourless oily liquid |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
Commercial chemical used to make fertilizers, explosives, dyestuffs and other chemicals. It is used in petroleum refining, ore processing, paper manufacturing, battery manufacturing, leather industries, printing and jewellery making. In some countries it is used in agriculture, as a desiccant on potatoes, flax and bulbs. It is used as a sanitizer for food processing and dairy facilities. [1] |
|
Handling considerations |
Keep only in original container. Wash skin thoroughly after handling. Wear protective gloves/protective clothing/eye protection/face protection. Wash contaminated clothing before reuse. Absorb spillage to prevent material damage. Store locked up. Store in corrosive resistant stainless steel container with a resistant inner liner. Dispose of contents/container to an approved waste disposal plant. IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. [1] |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
98.072 g/mol [3, 4] |
|
Bulk density/Specific gravity |
1.840 g/mL at 25 °C [4] 1.8302 g/cm3 [3], 1.835 g/cm3 at 20 °C [2] |
|
pH |
1 N solution = 0.3; 0.1 N solution = 1.2; 0.01 N solution = 2.1 [3] |
|
EC |
|
|
Melting point |
-42 - 10.5 °C [9] |
|
Boiling point |
163 - 360 °C @ 101.3 kPa [6] |
|
Flash point |
|
|
Flammability |
Non flammable (100%) [6] |
|
Vapour density |
3.4 (EPA, 1998) (Relative to Air) [3] |
|
Vapour pressure |
0.1 - 214 Pa @ 20 - 148.5 °C [6] 1 mmHg ( 146 °C) [4] |
|
Solubility in water |
Miscible with water with the generation of much heat and with contraction in volume [3] |
|
Solubility in organic solvents |
Miscible with alcohol with the generation of much heat and with contraction in volume [3] |
|
Hydrolysis |
|
|
Ionicity in water |
it is hygroscopic (absorbs water from the air); anion form in the environment [3] |
|
Surface tension |
51.7 mN/m at 50 deg C [3] |
|
Dispersion properties |
21 mPa.s at 25 deg C [4] |
|
Stability and reactivity |
|
|
Chemical stability |
Stable under recommended storage conditions [1] |
|
Reactivity hazards |
Stable under recommended storage conditions. [1] Very reactive, dissolves most metals; concentrated acid oxidizes, dehydrates, or sulfonates most organic compounds, often causes charring [2] |
|
Corrosivity |
strongly[1]; Concentrated acid is non-corrosive to lead and mild steel but dilute acid attacks most metals; The corrosivity is highly dependent on concentration, temperature, acid velocity, and acid impurities. [2] |
|
Polimerization |
|
|
Incompatibility with various substances |
Bases, halides, organic materials, carbides, fulminates, nitrates, picrates, cyanides, chlorates, alkali halides, zinc salts, permanganates, e.g. potassium permanganate, hydrogen peroxide, azides, perchlorates, nitromethane, phosphorous; Reacts violently with: cyclopentadiene, cyclopentanone oxime, nitroaryl amines, hexalithium disilicide, phosphorous(iii) oxide, powdered metals. [4] |
|
Special remarks on reactivity |
|
|
Physical, chemical and biological coefficients |
|
|
Koc |
|
|
Kow |
log Kow = -2.20 (est) [1, 2, 3] |
|
pKa |
pKa = 1.92 at 25 deg C [2, 3] |
|
Henry-constant |
9.9*10-15 atm-cu m/mole at 25 deg C [2] |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
||
|
Artificial pollution sources |
Sulfuric acid's production and use in the manufacture of fertilizers, explosives, dyestuffs and other chemicals and in petroleum refining, ore processing, paper manufacture and other uses may result in its release to the environment through various waste streams (SRC). Sulfuric acid droplets can form in the air when sulfur dioxide is released from the burning of coal, oil, and gas; the released sulfur dioxide slowly forms sulfur trioxide and then reacts with water in the air to form sulfuric acid. The occurrence of sulfuric acid in the environment comes mainly from the hydrolysis of sulfur oxides produced by combustion processes (natural and anthropogenic), wet deposition, generally as a mixture with nitrogen oxides and nitric acid and not from the manufacturing and use of the acid. In some countries, sulfuric acid is approved for agricultural use which will release the compound directly to the environment (SRC). [1] Sulfuric acid enters the waters in a variety of ways: in accidental spills from railcar derailments, in wastewaters from mining properties where sulphides are a part of the ore or the rock being mined, in wastewaters from the steel industry, from the atmosphere, and as a decomposition product of effluents containing, sulphur, thiosulphate, or other thionates [1]. |
|
|
General terrestrial fate |
If released to soil, it is expected to have high mobility since it is totally miscible in water. Its pKa is 1.92 at 25 deg C indicating that it will exist almost entirely in anion form in the environment (as the sulfate ion). Volatilization of sulfuric from moist soil surfaces is not expected to be an important fate process due to dissociation and a very low measured Henry's law constant of 9.9X10-15 atm-cu m/mole at 25 deg C. S. acid is not expected to volatilize from dry soil surfaces based upon its vapor pressure. In moist conditions, the sulfate anion may associate with cations including calcium, magnesium, and aluminum. The pKa of s. acid is 1.92 at 25 deg C, indicating that this compound will exist almost entirely in anion form in the environment (as the sulfate ion). S. acid dissociates readily in water to form sulfate ions and hydrated protons; at pH 3.92 the dissociation is 99%. S. acid is totally miscible in water suggesting a low Koc value and high mobility in soil. The ionization of s. acid also implies that s. acid, itself, will not adsorb on particulate material or soil surfaces. Volatilization of sulfuric from moist soil surfaces is not expected to be an important fate process due to dissociation (SRC) and a very low measured Henry's Law constant of 9.9X10-15 atm-cu m/mole at 25 deg C. S. acid is not expected to volatilize from dry soil surfaces(SRC) based upon a vapor pressure of 5.93X10-5 mm Hg at 25 deg C. Anaerobic bacteria in sediments and soil can reduce sulfate to sulfur and hydrogen sulfide. In moist conditions, the sulfate anion may associate with cations including calcium, magnesium, and aluminum. [2, 4] |
|
|
General aquatic fate |
If released into water, it is not expected to adsorb to suspended solids and sediment. Anaerobic bacteria in sediments and soil can reduce the sulfate ion to sulfur and hydrogen sulfide. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's ionization and Henry's Law constant. The pKa is 1.92 at 25 deg C, indicating that this compound will exist almost entirely in anion form in the environment (as the sulfate ion). It dissociates readily in water to form sulfate ions and hydrated protons; at pH 3.92 the dissociation is 99%. The ionization implies that it will not adsorb on particulate material. Ions do not volatilize from water, therefore, volatilization from surface waters is not expected to be an important fate process(SRC). Although nearly totally ionized at environmental pHs, it has a measurable Henry's Law constant of 9.9X10-15 atm-cu m/mole at 25 deg C. This Henry's Law constant also indicates that it is expected to be essentially nonvolatile from water surfaces. Its ionization implies that it will not accumulate in living tissues. Anaerobic bacteria in sediments can reduce sulfate to sulfur and hydrogen sulfide. In water, the sulfate anion may associate with cations including calcium, magnesium, and aluminum. [2, 4] |
|
|
General atmospheric fate |
If released to air, a vapor pressure of 5.93X10-5 mm Hg at 25 deg C indicates it will exist in both the vapor and particulate phases in the atmosphere. In the atmosphere it can react with other compounds to form sulfate aerosol particulates; for example, if ammonia is present, it will react to form ammonium sulfates. In air, submicron-sized sulfuric acid aerosol droplets rapidly take up water from the atmosphere. S. acid aerosols may be removed from the air by wet and dry deposition [2, 4]. |
|
|
General persistence and degradability |
|
|
|
Abiotic degradation and metabolites |
A rate constant of 1.5X10-14 cm3/molecule-sec at 25 deg C was approximated for the gas-phase reaction of hydroxyl radicals with a single hydrogen atom of sulfuric acid from the HSO4 radical; it was suggested that this reaction could be a sink for gaseous sulfuric acid in the atmosphere. Sulfuric acid in the atmosphere can react with other compounds to form sulfate aerosol particulates; for example, if ammonia is present, sulfuric acid will react to form ammonium sulphates [1] |
|
|
Biodegradation and metabolites |
Sulphuric acid is a simple inorganic substance, which will not biodegrade. The substance dissociates readily in water to form hydrogen ions and sulphate ions (at environmentally relevant pH) and is totally miscible with water. The hydrogen ions, although not degraded as such due to their elemental nature will react with and be neutralised by (OH) to form water. The sulphate ions are incorporated into the various mineral species present in the environment. No further information is necessary. [7] |
|
|
Bioconcentration |
Ionization implies that sulfuric acid will not accumulate in living tissues. [2] Sulphuric acid is a strong mineral acid (pKa =1.92) that dissociates readily in water to hydrogen ions and sulphate ions (at environmentally relevant pH) and is totally miscible with water. The resulting hydrogen ions and sulphate ions are naturally present in water/sediment and no bioaccumulation of these ions is predicted. [7] |
|
|
Volatilization |
The pKa of sulfuric acid is 1.92 at 25 deg C, indicating that this compound will exist almost entirely in anion form in the environment. Sulfuric acid dissociates readily in water to form sulfate ions and hydrated protons; at pH 3.92 the dissociation is 99%. Ions do not volatilize from water, therefore, volatilization from surface waters and moist soil is not expected to be an important fate process (SRC). Although nearly totally ionized at environmental pHs, sulfuric acid has a measurable Henry's Law constant of 9.9X10-15 atm-cu m/mole at 25 deg C. This Henry's Law constant also indicates that sulfuric acid is expected to be essentially nonvolatile from water surfaces. Sulfuric acid is not expected to volatilize from dry soil surfaces(SRC) based upon a vapor pressure of 5.93X10-5 mm Hg at 25 deg C [1, 4] |
|
| Photolysis |
Sulphuric acid is a strong mineral acid that will react with minerals and other soil constituents e.g. carbonates, liberating carbon dioxide, and forming the corresponding sulphate. Sulphate and hydrogen ions are not photolabile. Phototransformation will not occur. [7] Sulfuric acid and sulfuric acid aqueous solutions absorb at wavelengths >290 nm and, therefore, may be susceptible to direct photolysis by sunlight. [3] |
|
|
Hydrolysis |
Sulphuric acid is a strong mineral acid (pKa = 1.92) that dissociates readily in water to hydrogen ions and sulphate ions (at all environmentally relevant pH levels), and is totally miscible with water. Following dissociation, the hydrogen ion reacts with (OH) and yields water. At all environmentally relevant concentrations, the substance will therefore exist as the environmentally ubiquitous sulphate (SO4 -) anion and hydronium (H3O+) cation. No further studies on hydrolysis or additional information are required. [7] |
|
|
Soil adsorption and mobility |
The pKa of sulfuric acid is 1.92 at 25 deg C, indicating that this compound will exist almost entirely in anion form in the environment (as the sulfate ion). Sulfuric acid dissociates readily in water to form sulfate ions and hydrated protons; at pH 3.92 the dissociation is 99%. Sulfuric acid is totally miscible in water suggesting a low Koc value and high mobility in soil. The ionization of sulfuric acid also implies that sulfuric acid, itself, will not adsorb on particulate material or soil surfaces. During transport through the soil, sulfuric acid can dissolve some of the soil material, in particular carbonate |
|
|
ENVIRONMENTAL CONCENTRATIONS |
||
|
Measured data |
Sea water: Sulfuric acid was detected in 4 of 25 estuarine drainage systems in the US Gulf of Mexico [1] Arithmetic annual average atmospheric sulfate concentrations measured from 1964 to 1968 were 13.5 ug/ m3 for urban sites in the eastern United States and 6.4 ug/ m3 for urban sites in the western United States. A review of studies of the measurement of sulfuric acid or hydrogen ion (as sulfuric acid) in the United States from 1974 to 1986 indicated that the concentrations were generally below 5 ug/m3 [1]. Sulfuric acid has reportedly been detected in tobacco plants[1] |
|
|
ECOTOXICOLOGICAL INFORMATION |
||
|
General adverse effects on ecosystem |
||
|
Acute toxicity (LC50, EC50) |
||
|
Aquatic systems
Terrestrial systems |
LC50 16 mg/L (freshwater fish) [6] EC50 (48 h) 100 mg/L (aquatic invertebrates) [6] EC50 (72 h) 100 mg/L (aquatic algae and cyanobacteria)[6] |
|
|
Chronic toxicity (NOEC, LOEC) |
|
|
|
Aquatic systems
Terrestrial systems |
NOEC (65 days) 25 µg/L (fish) [6] NOEC 150 µg/L (aquatic invertebrates) [6] NOEC (37 days) 26 g/L (microorganisms) [6] NOEC (30 days) 30 g/L (microorganisms) [6]
|
|
|
HUMAN HEALTH EFFECTS and PROTECTION |
||
|
Routes of human exposures |
inhalation, ingestion, skin and/or eye contact [3] |
|
|
General effects |
irritation eyes, skin, nose, throat; pulmonary edema, bronchitis; emphysema; conjunctivitis; stomatis; dental erosion; eye, skin burns; dermatitis [3] |
|
|
Endocrine disruption |
Endocrine effects are not likely to occur in humans following exposure by any route. [8] Repeated exposure to H2SO4 suppresses macrophage effector/functional and biochemical activities important for maintaining pulmonary immunocompetence. [9] |
|
|
Mutagenicity Genotoxicity |
Because sulfuric acid is a direct-acting toxicant, developmental effects in mammals are not likely following exposure by any route. Genotoxicity is possible only if hydrogen ions come into direct contact with a cell. [8] |
|
|
Carcinogenicity |
There is sufficient evidence that occupational exposure to strong-inorganic-acid mists containing s. acid is carcinogenic. Overall evaluation: Occupational exposure to strong-inorganic-acid mists is carcinogenic to humans (Group 1) A2; Suspected human carcinogen. /Classification refers to s. acid contained in strong inorganic acid mists.). Strong inorganic acid mists containing s. acid are known to be human carcinogens based on sufficient evidence of carcinogenicity from studies in humans. [2, 3] |
|
|
Reprotoxicity |
Because sulfuric acid is a direct-acting toxicant, reproductive effects are not likely following exposure to sulfuric acid by any route. [8] |
|
|
Teratogenicity |
Because sulfuric acid is a direct-acting toxicant, developmental effects in mammals are not likely following exposure by any route. Genotoxicity is possible only if hydrogen ions come into direct contact with a cell. During inhalation or dermal exposure, hydrogen ions are not absorbed and distributed throughout the systemic circulation, and will not contact germ cells. However, developing organisms that are in direct contact with the environment (e.g., insects, amphibians) could be adversely affected if there are sufficient levels of sulfuric acid to result in decreased pH. [8] |
|
|
Skin, eye and respiratory irritations |
Very irritative [4] |
|
|
Metabolism: absorption, distribution & excretion |
The substance can be absorbed into the body by inhalation of its aerosol. [3]
|
|
|
Exposure limits |
EU-OEL: 0.05 mg/m³ mint TWA [4] |
|
|
Drinking water MAC |
0.1 mg/m3 [4] |
|
|
Other information |
|
|
|
Animal toxicity data |
||
|
Acute toxicity (LD50) |
LD50 2 140 mg/kg bw (rat) (oral) [6] LC50 (8 h) 600 mg/m³ air (mouse) (inhalation) [6] LC50 (4 h) 850 mg/m³ air (mouse) (inhalation) [6] |
|
|
Chronic toxicity (NOEL, LOEL) |
LOAEC (rat): 300 µg/m³ air (inhalation) [6] |
|
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
||
| REACH/CLP |
Danger! According to the harmonised classification and labelling (CLP00) approved by the European Union, this substance causes severe skin burns and eye damage. Acoording to REACH registration dossiers notifications: H314: Causes severe skin burns and eye damage Additionally, the classification provided by companies to ECHA in REACH registrations identifies that this substance is toxic if inhaled. Additionally, the classification provided by companies to ECHA in CLP notifications identifies that this substance causes serious eye damage, may be corrosive to metals and may cause respiratory irritation. Acoording to CLP notifications: H314: Causes severe skin burns and eye damage. H318: Causes serious eye damage. H290: May be corrosive to metals. H335: May cause respiratory irritation. H350: May cause cancer. |
|
|
EINECS regulation |
̵ |
|
|
OSHA regulations etc. |
HE10, HE11, HE3, HE14 1 mg/m3 [5] |
|
|
OTHER INFORMATION, SPECIAL REMARKS |
||
|
Classification and proposed labelling with regard to toxicological data |
|
|
|
CREATED, LAST UPDATE |
||
|
Created |
6th of April, 2018 |
|
|
Last update |
25th of May, 2020 |
|
|
REFERENCES |
||
|
[1]. TOXNET. Toxicology Data Network. Available from: https://toxnet.nlm.nih.gov/cgibin/sis/search2/f?./temp/~5r0Lx6:1. [Accessed: 2018. 06. 12.] [2] International Programme on Chemical Safety (IPCS INCHEM) (2001) Screening Information Data Set (SIDs) for High Production Volume Chemicals (Sulphuric acid) SIDS Initial Assessment Report for 11th SIA, Available from: http://www.inchem.org/documents/sids/sids/7664939.pdf [Accessed: 2018. 04 12.] [3] Pubchem. Open Chemistry database, Available from: https://pubchem.ncbi.nlm.nih.gov/compound/1118. [Accessed: 2018. 04 12.] https://pubchem.ncbi.nlm.nih.gov/compound/1118#section=Environmental-Fate-Exposure-Summary [Accessed: 2020. 05. 25.] [4] National Insitute of Health, US National Library of Medicine (NIH-NLM) WEBWISER. Available from:https://webwiser.nlm.nih.gov/WebWISER/getSubstanceData.do?substanceId=316&displaySubstanceName=Battery%20acid&STCCID=&UNNAID=&selectedDataMenuItemID=76&catId=115. [Accessed: 2018. 04. 12.] [5] OSHA. Occupational Safety and Health Administration, Available from: https://www.osha.gov/dts/chemicalsampling/data/CH_268700.html. [Accessed: 2018. 04 12.] [6] European Chemicals Agency (ECHA). Substance information. Available from: https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.028.763, [Accessed: 2018. 05. 06] [7] European Chemicals Agency (ECHA). Sulphuric acid. https://echa.europa.eu/hu/registration-dossier/-/registered-dossier/16122/5/2/5, [Accessed: 2020. 05. 25] [8] U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry, TOXICOLOGICAL PROFILE FOR SULFUR TRIOXIDE AND SULFURIC ACID (1998) https://www.atsdr.cdc.gov/toxprofiles/tp117.pdf, [Accessed: 2020. 05. 25] [9] J T Zelikoff, M P Sisco, Z Yang, M D Cohen, R B Schlesinger (1994) Immunotoxicity of Sulfuric Acid Aerosol: Effects on Pulmonary Macrophage Effector and Functional Activities Critical for Maintaining Host Resistance Against Infectious Diseases, Toxicology, 92 (1-3):269-86, doi: 10.1016/0300-483x(94)90183-x. |
||