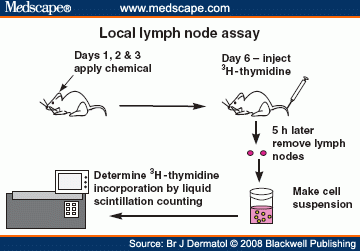
The basic principle underlying the LLNA is that sensitisers induce a primary proliferation of lymphocytes in the lymph node draining the site of chemical application. This proliferation is proportional to the dose applied (and to the potency of the allergen) and provides a simple means of obtaining an objective, quantitative measurement of sensitisation. The LLNA assesses this proliferation as a doseresponse in which the proliferation in test groups is compared to that in vehicle treated controls. The ratio of the proliferation in treated groups to that in vehicular controls, termed the Stimulation Index, is determined, and must be at least three before a test substance can be further evaluated as a potential skinsensitiser. The methods described here are based on the use of radioactive labelling to measure cell proliferation. However, other endpoints for assessment of proliferation may be employed provided there is justification and appropriate scientific support, including full citations and description of the methodology.
A minimum of four animals is used per dose group, with a minimum of three concentrations of the test substance, plus a negative control group treated only with the vehicle for the test substance, and a positive control, as appropriate. In those cases in which individual animal data are to be collected, a minimum of five animals per dose group are used. Dose and vehicle selection should be based on the recommendations given in reference (2). Doses are selected from the concentration series 100%, 50%,25%, 10%, 5%, 2.5%, 1%, 0.5% etc. Existing acute toxicity and dermal irritation data should be considered, where available, in selecting the three consecutive concentrations so that the highest concentration maximises exposure whilst avoiding systemic toxicity and excessive local skin irritation. Except for absence of treatment with the test substance, animals in the control groups should behandled and treated in a manner identical to that of animals in the treatment groups. The vehicle should be selected on the basis of maximising the test concentrations and solubilitywhilst producing a solution/suspension suitable for application of the test substance. In order ofpreference, recommended vehicles are acetone/olive oil (4:1 v/v), dimethylformamide, methyl ethylketone, propylene glycol and dimethyl sulphoxide, but others may be used if sufficient scientificrationale is provided. In certain situations it may be necessary to use a clinically relevant solvent or thecommercial formulation in which the test substance is marketed as an additional control. Particular careshould be taken to ensure that hydrophilic materials are incorporated into a vehicle system, which wets theskin and does not immediately run off. Thus, wholly aqueous vehicles are to be avoided.
A single cell suspension of lymph node cells (LNC) either from pooled treatment groups orbilaterally from individual animals is prepared by gentle mechanical disaggregation through 200 μm-meshstainless steel gauze. Lymph node cells are washed twice with an excess of PBS and precipitated with 5% trichloroacetic acid (TCA) at 4°C for 18h. Pellets are either re-suspended in 1 mL TCA and transferred to scintillation vials containing 1.0 mL of scintillation fluid for 3H-counting, or transferred directly to gamma counting tubes for 125I-counting.
Incorporation of 3H-methyl thymidine is measured by β-scintillation counting as disintegrations per minute (DPM). Incorporation of 125I-iododeoxyuridine is measured by 125I-counting and also is expressed as DPM. Depending on the approach used, the incorporation will be expressed as DPM/treatment group (pooled approach) or DPM/animal (individual approach).
Results are expressed as the Stimulation Index (SI). When using the pooled approach, the SI is obtained by dividing the pooled radioactive incorporation for each treatment group by the incorporation of the pooled vehicle control group; this yields a mean SI. When using the individual approach, the SI is derived by dividing the mean DPM /mouse within each test substance group and the positive control group by the mean DPM/mouse for the solvent/vehicle control group. The average SI for vehicle treated controls is then 1.