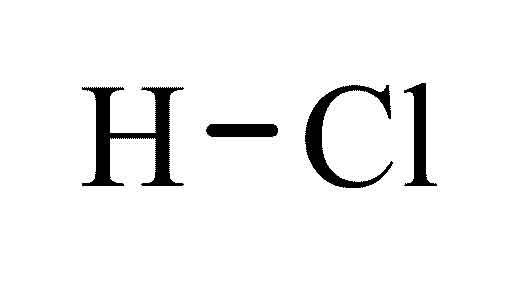CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
Hydrochloric acid |
|
Synonyms |
Hydrogen chloride; Muriatic acid; 7647-01-0; Chlorohydric acid; etc [1] |
|
IUPAC name |
chlorane [1] |
|
CAS No |
7647-01-0 and 7698-05-7 [1] |
|
REACH registration number |
- |
|
EC No |
231-595-7 and 231-715-8 [1] |
|
Molecular formula |
HCl or ClH[1]
|
|
Substance group/chemical family |
Mono constituent substance/inorganic acid |
|
Appearance Physical state
Odour Form Colour |
irritating, sharp |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
Use in chemical industry, manufacture of fertilizers, hydrolysing of starch and proteins, in the preparation of various food products, pickling and cleaning of metal products, refining ore, leather deliming / tanning agents, oil- and gas-well treatment, textile scouring agents, removing scale from boilers and heat-exchange equipment, in the brewing industry, water treatment (pH control etc.), as laboratory reagent. [2] |
|
Handling considerations |
See the Precautionary Statement Codes: P234, P260, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P312, P321, P363, P390, P403+P233, P404, P405, P410+P403, and P501 [1] |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
36.458 g/mol [1] |
|
Bulk density/Specific gravity |
1.19 g/mL (38% HCl concentration) (@ 20 °C and 1 atmosphere (101.325 kPa). [7] |
|
pH |
|
|
EC |
1.7X10-7 1/Ohm m at 158.94 K; 3.5X10-7 1/Ohm m at 185.56 K [3] |
|
Melting point |
-114.2°C [1, 4] |
|
Boiling point |
-85.1°C [1, 4] |
|
Flash point |
|
|
Flammability |
Non flammable (100%) [5] |
|
Vapour density |
1.268 (Air = 1.000) [1, 3, 4] |
|
Vapour pressure |
4 620 kPa @ 25 °C [5] |
|
Solubility in water |
823 g/L at 0°C [2] |
|
Solubility in organic solvents |
methanol: 47.0 g/100 g (20°C); etanol: 1.0 g/100 g (20°C) |
|
Hydrolysis |
HCl + H2O = H3O+ + Cl– [2] |
|
Ionicity in water |
ionization potential: 12.74 eV [1] |
|
Surface tension |
23 mN/cm at 118.16 K [3] |
|
Dispersion properties |
|
|
Stability and reactivity |
|
|
Chemical stability |
instable [1]. Its aqueous solution (called hydrochloric acid) possesses strong acidity, and reacts with most metals producing explosive hydrogen gas. Hydrogen chloride is readily dissociated in water into hydrated protons and chloride ion. [2] |
|
Reactivity hazards |
very reactive[1] |
|
Corrosivity |
Corrosive [1] |
|
Polimerization |
Aldehydes and epoxides in the presence of hydrochloric acid cause violent polymerization. Alcohol and glycols in the presence of hydrochloric acid lead to dehydration reactions. [1, 3] |
|
Incompatibility with various substances |
|
|
Special remarks on reactivity |
high thermal stability. [1] |
|
Physical, chemical and biological coefficients |
|
|
Koc |
|
|
Kow log Pow |
0.25 [1, 2, 4] |
|
pKa |
|
|
Henry-constant |
2.04X10+6 mol/L atm (4.90X10-10 atm-cu m/mol) /Hydrochloric acid/ [3] |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
Hydrogen chloride and hydrochloric acid production and use as a chemical intermediate and laboratory reagent, production of metals, refining, leather tanning, rubber production, in food processing, disinfectant and sanitizerand as a pharmaceutical aid may result in the release of hydrogen chloride or hydrochloric acid to the environment through various waste streams. Hydrogen chloride's use in oil and gas well treatment, formation during the burning of many plastics and combustion of fossil fuel use as a fungicide and slimicide and use in hydraulic fracturing to dissolve minerals and initiate cracks in the rocks will result in its direct release to the environment. [3] |
|
General terrestrial fate |
If released to soil, it dissociates into chloride and hydronium ions in moist soil. Volatilization from moist soil surfaces is not expected to be an important fate process based upon a estimated Henry's Law constant Hydrogen chloride will evaporate from dry soil surfaces. [3] |
|
General aquatic fate |
If released to water, hydrogen chloride dissociates readily in water to chloride and hydronium ions. The dissociation results in a decreasing of the pH of the water. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's Henry's Law constant. [3] |
|
General atmospheric fate |
Hydrochloric acid is found in the gases that evolve from volcanoes. Hydrochloric acid is also found in the digestive tract of most mammals. If released to air, hydrogen chloride will be removed by rainfall. Anhydrous hydrogen chloride released into the air will be in the vapor form. Once released to the environment it will react with atmospheric moisture and standing water to form hydrochloric acid. Hydrogen chloride is removed from air by wet deposition as chloride salts with an atmospheric lifetime of 1-5 days. [3] |
|
General persistence and degradability |
|
|
Abiotic degradation and metabolites |
Hydrogen chloride is removed from air by wet deposition as chloride salts with an atmospheric lifetime of 1-5 days. Hydrogen chloride dissociates readily in water to chloride and hydronium ions, decreasing the pH of the water. [3] |
|
Biodegradation and metabolites |
|
|
Bioconcentration |
Hydrogen chloride dissociates readily in water to chloride and hydronium ions. Therefore, hydrogen chloride does not accumulate in the aquatic organisms. [3] |
|
Volatilization |
The Henry's Law constant for hydrogen chloride is 2.04X10+6 mol/L atm (4.90X10-10 m3 atm/mol). This Henry's Law constant indicates that hydrogen chloride is expected to be essentially non-volatile from water and moist soil surfaces. Hydrogen chloride will evaporate from dry soil surfaces [3]. |
|
Photolysis |
|
|
Hydrolysis |
|
|
Soil adsorption and mobility |
Hydrogen chloride dissociates into chloride and hydronium ions in moist soil. [3] |
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
The UNEP (United Nations Environment Programme) reported the mean, the 10th-pecentile and the 90th-percentile of chloride concentrations in 77 rivers were 41.1, 1.1 and 64.8 mg/L, respectively. This global water quality monitoring was conducted in North America, South-America, Asia, Africa, Europe and Oceania. The chloride concentration is tightly related to the geological parameters and human activities and chloride is also extensively distributed into the environment. Thus the chloride concentration is not only related to the release of hydrogen chloride/hydrochloric acid into the environment. Regarding the H+ concentration measured as pH, UNEP reported that the average annual pH values were between 6.5 and 8.3. [2] |
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems
Terrestrial systems |
The hazard of hydrochloric acid for the environment is caused by the proton (pH effect). For this reason the effect of hydrochloric acid on the organisms depends on the buffer capacity of the aquatic ecosystem. Also the variation in acute toxicity for aquatic organisms can be explained for a significant extent by the variation in buffer capacity of the test medium. For example, LC50 values of acute fish toxicity tests varied from 4.92 to 282 mg/L. [3] Microorganisms: EC50 / LC50: 230 µg/L [5] |
|
Chronic toxicity (NOEC, LOEC) |
No long-term or chronic toxicity data on invertebrates have been reported. No long-term or chronic toxicity data on fish have been reported. [2] |
|
Aquatic systems
Terrestrial systems |
There is only one study available on acute and chronic toxicity to unicellular green algae. For Selenastrum capricornutum, 72h EC50 and NOEC based on growth rate are pH 5.3 (equivalent to a substance concentration of 0.492 mg/L) and pH 6.0 (0.097 mg/L), respectively. A long-term toxicity test has been performed on Selenastrum capricornutum. The 72h NOEC on biomass and growth rate was pH 6.0 (0.097 mg/L). [2] |
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
Serious local effects by all routes of exposure. The substance can be absorbed into the body by inhalation. [4] |
|
General effects |
Effects of short-term exposure: Rapid evaporation of the liquid may cause frostbite. The substance is corrosive to the eyes, skin and respiratory tract. Inhalation of this gas may cause asthma-like reactions (RADS). Exposure could cause asphyxiation due to swelling in the throat. Inhalation of high concentrations may cause lung oedema, but only after initial corrosive effects on the eyes and the upper respiratory tract have become manifest. Inhalation of high concentrations may cause pneumonitis. Effects of long-term or repeated exposure: Repeated or prolonged inhalation may cause effects on the teeth. This may result in tooth erosion. The substance may have effects on the upper respiratory tract and lungs. This may result in chronic inflammation of the respiratory tract and reduced lung function. Mists of this strong inorganic acid are carcinogenic to humans. [4] |
|
Endocrine disruption |
|
|
Mutagenicity |
In bacteria: negative by AMES, but Rec Assay are positive [2] Mammals cell: Chromosome aberration (Sister Chromatide exchanged so had negative and positive results too (mouse lymphoma) in different studies. Mutagenic effects of hydrochloric acid were obtained in the sex linked recessive lethal study with D. melanogaster by inhalation of vapour or larval feeding of the solution (only one dose level tested). [2] |
|
Carcinogenicity |
There is inadequate evidence for the carcinogenicity in humans of hydrochloric acid. There is inadequate evidence for the carcinogenicity in experimental animals of hydrochloric acid. Hydrochloric acid is not classifiable as to its carcinogenicity to humans (Group 3). [3] NOAEC 15 mg/m³ (chronic, rat) (Inhalation route) [5] |
|
Reprotoxicity |
|
|
Teratogenicity |
|
|
Skin, eye and respiratory irritations
Metabolism: absorption, distribution & excretion
|
very irritative. An aqueous solution (4%) of hydrogen chloride was slightly irritating, and a 10% solution was determined to be 'Irritating to skin' in human volunteer experiments. [1, 2, 3]
Increased urinary excretion of the chloride ion were observed in rats and dogs intravenously injected 0.15 M hydrochloric acid solution. |
|
Exposure limits |
TLV: 2 ppm as STEL; A4 (not classifiable as a human carcinogen). |
|
Drinking water MAC |
3 mg/m3; 2 ppm [4] |
|
Other information |
|
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
Inhalation route: LC50 (30 min) 8.3 mg/L air (rat) LC50 (30 min) 4 701 ppm (rat) LC50 (5 min) 45.6 mg/L air (rat) LC50 (5 min) 40 989 ppm (rat) Most conservative value: LC50 7 051 mg/m³ [5] |
|
Chronic toxicity (NOEL, LOEL) |
Inhalation route - systemic effects: NOAEC 30 mg/m³ (subchronic, rat) Inhalation route - local effects: NOAEC 15 mg/m³ (subchronic, rat) [5] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
| REACH/CLP |
Danger! According to the classification provided by companies to ECHA in REACH registrations this substance causes severe skin burns and eye damage, is toxic if inhaled, may damage fertility or the unborn child, causes serious eye damage, may cause damage to organs through prolonged or repeated exposure, may be corrosive to metals and may cause respiratory irritation. According to REACH registrations: H335: May cause respiratory irritation. H314: Causes severe skin burns and eye damage. H331: Toxic if inhaled. H290: May be corrosive to metals. H318: Causes serious eye damage. According to CLP notifications: H335: May cause respiratory irritation. H314: Causes severe skin burns and eye damage. H331: Toxic if inhaled. H290: May be corrosive to metals. H318: Causes serious eye damage. H302: Harmful if swallowed. H311: Toxic in contact with skin. |
|
EINECS regulation |
listed |
|
OSHA regulations etc. |
HE3, HE4, HE11, HE14 Permissible Exposure Limit: Ceiling value: 5 ppm (7 mg/cu m) [1, 6] |
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
CREATED, LAST UPDATE |
|
|
Created: Last updated: |
12. 04. 2018 25. 09. 2019 |
|
REFERENCES |
|
|
[1] Open Chemistry database, PUBCHEM, Available from: https://pubchem.ncbi.nlm.nih.gov/compound/313#section=Top. [Accessed: 2018.04.12.] https://pubchem.ncbi.nlm.nih.gov/compound/hydrochloric_acid#section=GHS-Classification [Accessed: 2018.06.20.] [2] International Program on Chemical Safety INCHEM. OECD SIDS Available from: http://www.inchem.org/documents/sids/sids/7647010.pdf. [Accessed : 2018.04.12.] [3] TOXNET. Toxicology Data Network. Available from: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+7647-01-0. [Accessed:2018.04.12.] [4] International Program on Chemical Safety INCHEM, ICSC Available from: http://www.inchem.org/documents/icsc/icsc/eics0163.htm [Accessed : 2018.06.18.] [5] European Chemicals Agency - ECHA Available from: https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.028.723 [Accessed : 2018. 06. 18.] [6] OSHA. Occupational Safety and Health Administration. Available from: https://www.osha.gov/dts/chemicalsampling/data/CH_246300.html. [Accessed: 2018.04.12.] https://www.osha.gov/chemicaldata/chemResult.html?recNo=620 [Accessed: 2018.06.20.] [7] Wikipedia, Hydrochloric acid: https://en.wikipedia.org/wiki/Hydrochloric_acid [Accessed: 2019.09.25.] |
|